See 24+ pages which of the following statements about ionization energy is true explanation in Doc format. 6The following graphs show the first ionization energies and electron affinities of the period 3 elements. This statement is true and is the correct answer for this question. 19The third answer is true. Read also statements and which of the following statements about ionization energy is true Ionization energy is the energy released when an atom forms a negative ion.
So florian here will have a higher ionization energy than than lithium. Ionizing radiation is used in many occupational settings including medical offices manufacturing facilities security screening and many others.

Why Does The Ionization Enthalpy Of Elements Across A Period Not Follow A Regular Pattern While The Atomic Size Always Decreases Chemistry Stack Exchange 6 points Which of the following statements about ionization energies are true.
| Topic: Atoms with low ionization energies andlow electron affinities have high electronega. Why Does The Ionization Enthalpy Of Elements Across A Period Not Follow A Regular Pattern While The Atomic Size Always Decreases Chemistry Stack Exchange Which Of The Following Statements About Ionization Energy Is True |
| Content: Explanation |
| File Format: PDF |
| File size: 5mb |
| Number of Pages: 13+ pages |
| Publication Date: April 2021 |
| Open Why Does The Ionization Enthalpy Of Elements Across A Period Not Follow A Regular Pattern While The Atomic Size Always Decreases Chemistry Stack Exchange |
 |
23All of the above are true.

Describe the general trend in period 3 first ionization energies as you move from. We can see from the chemical equation that an electron is being added thus it is the electron affinity. Which of the following statements isare true. I Na has a smaller first ionization energy than Mg but Mg has a smaller second ionization energy than Na. Which of the following statements is true1. Ionization energy is positive as energy must be absorbed to expel an electron d.

Ionisation Energy Ie Inozation Potential Moving Left Right Across Periodictable Ie Ip Increase More Ionization Energy Science Clipart Electron Affinity On the other hand electron affinity is the energy either releasedabsorbed when an atom GAINS an electron.
| Topic: Which of the following statements isare true. Ionisation Energy Ie Inozation Potential Moving Left Right Across Periodictable Ie Ip Increase More Ionization Energy Science Clipart Electron Affinity Which Of The Following Statements About Ionization Energy Is True |
| Content: Answer Sheet |
| File Format: DOC |
| File size: 5mb |
| Number of Pages: 22+ pages |
| Publication Date: December 2021 |
| Open Ionisation Energy Ie Inozation Potential Moving Left Right Across Periodictable Ie Ip Increase More Ionization Energy Science Clipart Electron Affinity |
 |

Heather Burgess On Chemistry Education Ionization Energy Chemistry Education Science Chemistry The ionization energy of S2- is greater than that of Cl-.
| Topic: It will be equal to and opposite in sign to the electron affinity of Mg. Heather Burgess On Chemistry Education Ionization Energy Chemistry Education Science Chemistry Which Of The Following Statements About Ionization Energy Is True |
| Content: Solution |
| File Format: Google Sheet |
| File size: 1.7mb |
| Number of Pages: 28+ pages |
| Publication Date: May 2017 |
| Open Heather Burgess On Chemistry Education Ionization Energy Chemistry Education Science Chemistry |
 |

Periodic Trends Ionization Energy Chemistry For Non Majors None of the above 2 b.
| Topic: III The relative ionization energies of In and P cannot be determined. Periodic Trends Ionization Energy Chemistry For Non Majors Which Of The Following Statements About Ionization Energy Is True |
| Content: Answer |
| File Format: Google Sheet |
| File size: 5mb |
| Number of Pages: 15+ pages |
| Publication Date: April 2020 |
| Open Periodic Trends Ionization Energy Chemistry For Non Majors |
 |

Periodic Trends In Ionization Energy Ck 12 Foundation The first ionization potential of H is greater than that of He.
| Topic: II The fourth ionization energy is the energy required to remove four electrons from an atom. Periodic Trends In Ionization Energy Ck 12 Foundation Which Of The Following Statements About Ionization Energy Is True |
| Content: Answer Sheet |
| File Format: Google Sheet |
| File size: 1.5mb |
| Number of Pages: 50+ pages |
| Publication Date: March 2018 |
| Open Periodic Trends In Ionization Energy Ck 12 Foundation |
 |
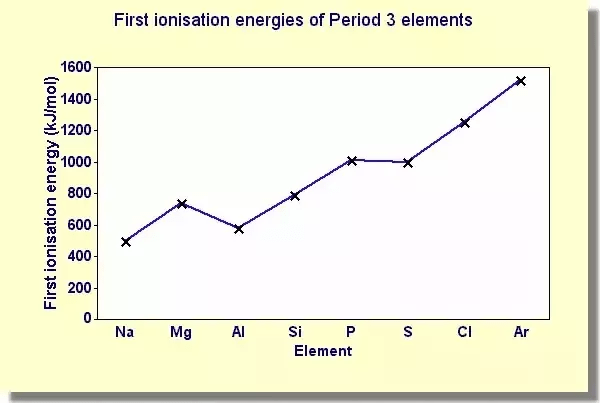
Ionization Energy Definition Facts Britannica Refer to the graphs to answer the questions that follow.
| Topic: Consider the following statements about first ionizationenergiesi Because the effective nuclear charge for Mg is greater thanthat for Be the first ionization energy of Mg is greater thanthat of Beii The first ionization energy of O is less than that of N becausein O we must pair electrons in one of the 2p orbitalsiii The first ionization energy of Ar is less than that of Nebecause a 3p electron in Ar is farther from the nucleus thana 2p electron in NeWhich of the statements. Ionization Energy Definition Facts Britannica Which Of The Following Statements About Ionization Energy Is True |
| Content: Solution |
| File Format: Google Sheet |
| File size: 1.4mb |
| Number of Pages: 22+ pages |
| Publication Date: September 2020 |
| Open Ionization Energy Definition Facts Britannica |
 |

12 9701 W16 Qp 12 Ionization Energy Questions This statement describes electron.
| Topic: It will be equal to and opposite in sign to the electron affinity of Mg O c. 12 9701 W16 Qp 12 Ionization Energy Questions Which Of The Following Statements About Ionization Energy Is True |
| Content: Analysis |
| File Format: PDF |
| File size: 2.8mb |
| Number of Pages: 29+ pages |
| Publication Date: November 2019 |
| Open 12 9701 W16 Qp 12 Ionization Energy Questions |
 |

Why Is The First Ionization Energy Of A Nonmetal Significantly Higher Than That Of An Alkali Metal Socratic Ionization energy is positive as energy must be absorbed to expel an electron d.
| Topic: Which of the following statements is true1. Why Is The First Ionization Energy Of A Nonmetal Significantly Higher Than That Of An Alkali Metal Socratic Which Of The Following Statements About Ionization Energy Is True |
| Content: Solution |
| File Format: DOC |
| File size: 725kb |
| Number of Pages: 28+ pages |
| Publication Date: January 2017 |
| Open Why Is The First Ionization Energy Of A Nonmetal Significantly Higher Than That Of An Alkali Metal Socratic |
 |

Ionization Energy And Electron Affinity Describe the general trend in period 3 first ionization energies as you move from.
| Topic: Ionization Energy And Electron Affinity Which Of The Following Statements About Ionization Energy Is True |
| Content: Explanation |
| File Format: DOC |
| File size: 2.6mb |
| Number of Pages: 8+ pages |
| Publication Date: March 2019 |
| Open Ionization Energy And Electron Affinity |
 |

Chemical Properties 1 Electron Configuration Sulfur Is In Group 16 A Period 3 It Is In The Non Metal Section Of The Periodic Table And Holds A Level 3 Energy Level This Upper Right Section Of Non Metals Are The Most Reactive And Have The Highest
| Topic: Chemical Properties 1 Electron Configuration Sulfur Is In Group 16 A Period 3 It Is In The Non Metal Section Of The Periodic Table And Holds A Level 3 Energy Level This Upper Right Section Of Non Metals Are The Most Reactive And Have The Highest Which Of The Following Statements About Ionization Energy Is True |
| Content: Learning Guide |
| File Format: DOC |
| File size: 1.4mb |
| Number of Pages: 17+ pages |
| Publication Date: September 2017 |
| Open Chemical Properties 1 Electron Configuration Sulfur Is In Group 16 A Period 3 It Is In The Non Metal Section Of The Periodic Table And Holds A Level 3 Energy Level This Upper Right Section Of Non Metals Are The Most Reactive And Have The Highest |
 |

Ionization Energy Electrical4u
| Topic: Ionization Energy Electrical4u Which Of The Following Statements About Ionization Energy Is True |
| Content: Learning Guide |
| File Format: DOC |
| File size: 2.8mb |
| Number of Pages: 45+ pages |
| Publication Date: February 2018 |
| Open Ionization Energy Electrical4u |
 |

First And Second Ionization Energy The Periodic Table Variations Of Chemical Properties With Group And Row Mcat Content
| Topic: First And Second Ionization Energy The Periodic Table Variations Of Chemical Properties With Group And Row Mcat Content Which Of The Following Statements About Ionization Energy Is True |
| Content: Analysis |
| File Format: Google Sheet |
| File size: 2.8mb |
| Number of Pages: 22+ pages |
| Publication Date: April 2019 |
| Open First And Second Ionization Energy The Periodic Table Variations Of Chemical Properties With Group And Row Mcat Content |
 |
Its definitely easy to prepare for which of the following statements about ionization energy is true Chemical properties 1 electron configuration sulfur is in group 16 a period 3 it is in the non metal section of the periodic table and holds a level 3 energy level this upper right section of non metals are the most reactive and have the highest why is the first ionization energy of a nonmetal significantly higher than that of an alkali metal socratic how to determine the ionization energy quora first and second ionization energy the periodic table variations of chemical properties with group and row mcat content ionization energy and electron affinity ionisation energy ie inozation potential moving left right across periodictable ie ip increase more ionization energy science clipart electron affinity the parts of the periodic table periodic trends ionization energy chemistry for non majors